09-April-2025
So, what are the differences in particle size requirements for α-alumina as an abrasive in these four specific applications?
After polishing an automotive paint surface, it is crucial to avoid secondary scratches caused by the abrasive. Therefore, α-alumina used in this application should have a relatively rounded shape—typically spherical or columnar—with minimal sharp edges. The particles should also be uniform in size and dispersion, avoiding agglomeration as much as possible.
Depending on the level of paint surface damage, different polishing stages are required: coarse polishing, medium polishing, and fine polishing.
Generally speaking, larger α-alumina particle sizes provide stronger cutting ability but result in a rougher finish, while smaller particle sizes offer better surface smoothness but reduced cutting efficiency. As such, for automotive paint polishing, the recommended particle size ranges for α-alumina are:
- Coarse polishing: 45–60 μm
- Medium polishing: 5–5.9 μm
- Fine polishing: 1–2 μm

Sapphire, which is single-crystal alumina, has experienced increasing demand as a substrate material due to the rapid development of optoelectronic technology and the expansion of LED components. Sapphire has become one of the most important substrate materials, meeting substantial domestic and international market needs. When used as a substrate, sapphire requires an exceptionally flat surface, making its polishing process a current research hotspot.
During the polishing of sapphire, the reaction between the aluminum on the sapphire surface and the hydroxyl groups in the polishing slurry forms a pumice-like hydration layer with a Mohs hardness of 3. With smaller abrasive particle sizes, the particles might not be able to fully penetrate the hydration layer, meaning that the polishing abrasives do not effectively participate in the mechanical polishing, which results in a lower material removal rate. As the alumina particle size increases to 360 nm, the material removal rate gradually increases, and the surface roughness reaches its lowest point. With larger alumina particles, a greater number of effective abrasives participate in the polishing process, enhancing the material removal rate until a dynamic balance between mechanical and chemical polishing is achieved. At this point, all damage on the sapphire wafer is removed, resulting in a flat surface and relatively high quality with low surface roughness.
When the abrasive particle size increases to 560 nm, the material removal rate peaks; however, the surface roughness also increases significantly, leading to a suboptimal polishing effect. This is because larger polishing abrasives enhance the mechanical polishing effect, causing the material removal depth to increase and, consequently, inflicting more severe damage on the wafer. Furthermore, when the particle size reaches 1.5 μm, the stability of the polishing slurry deteriorates, resulting in slight stratification during the polishing process, which leads to poorer dispersion of the abrasives and an unsatisfactory polishing effect.
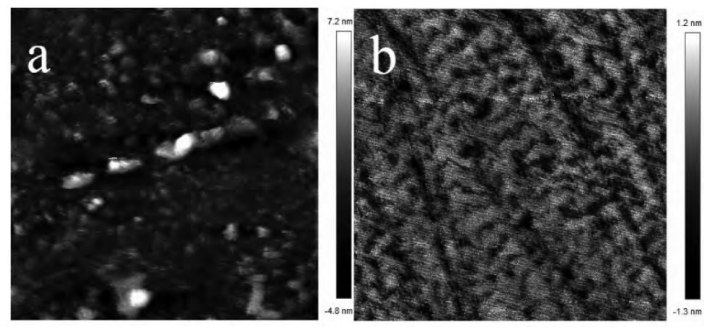
The figure above shows AFM images of the sapphire wafer surface before and after polishing with 360 nm spherical α-alumina. It can be observed that before polishing, the sapphire wafer exhibited an uneven surface with many rough peaks; after polishing, the scratch marks were greatly improved, with many of the rough peaks removed. The surface became smooth and flat, with only minimal microscopic undulations.
For silicon wafer polishing, it is best to process the α-alumina powder into a flat, plate-like shape. This allows the particles to closely conform to the workpiece surface during grinding, creating a sliding abrasive effect that minimizes scratches from sharp edges. Additionally, the grinding pressure is evenly distributed over the particle surfaces, making them less prone to fracturing. These factors combine to improve both the grinding efficiency and the surface finish, thereby reducing the grinding time, significantly enhancing productivity, decreasing wear on the polishing equipment, saving labor and processing costs, and pushing the yield rate above 90%.
When the material being polished is a silicon carbide wafer (Mohs hardness 9.2), the relatively lower Mohs hardness of α-alumina (8.8) necessitates mixing diamond micropowder (Mohs hardness 10) with the α-alumina micropowder to produce an effective abrasive. However, due to the irregular morphology and high hardness of diamond abrasives, mechanical polishing typically leaves the silicon carbide wafer with a surface roughness between 10 nm and 20 nm; under microscopic observation, several scratches of varying depths can be seen. This indicates that the damage layer is relatively deep, which makes it difficult for subsequent chemical mechanical polishing (CMP) to completely remove the layer created by mechanical polishing (MP).
To address this, the α-alumina micropowder should be produced with a particle size in the range of 0.5 μm to 5 μm and a specific surface area of 100–250 m²/g. This optimized particle size helps lower the surface roughness and reduces the depth of the subsurface damage layer on the silicon carbide wafer, thereby mitigating the scratch and damage issues introduced by the diamond micropowder. As a result, the wafer surface exhibits minimal scratches, a thin damage layer, and low roughness—conditions that are ideal for subsequent chemical mechanical polishing. If the particle size of the α-alumina micropowder is larger than this range, it tends to cause scratches and chipping. Conversely, if the particle size is smaller than the specified range, it fails to effectively counteract the damage caused by the diamond micropowder.

Polishing powders for glass lenses are typically composed of cerium oxide, aluminum oxide, silicon dioxide, iron oxide, zirconium oxide, chromium oxide, and other components. Due to the high chemical reactivity of cerium oxide with silicate glass and its comparable hardness, cerium oxide is widely used as the abrasive material in glass lens polishing. In some formulations of cerium oxide polishing powders, a small amount of the harder α-alumina is added, which enhances both the material removal rate and wear resistance. Since the Mohs hardness of silicate glass generally falls between 6 and 6.5—and given that the proportion of α-alumina is relatively low—there is no stringent requirement on the microscopic structure of α-alumina crystals; normally, as long as the particle size is not too large and the edges are not overly sharp, the specifications are met.
It is also noteworthy that doping aluminum into the surface of cerium oxide can significantly increase its polishing erosion rate on optical glass. This is achieved by mechanically ball-milling hydrated cerium carbonate with aluminum nitrate and ammonia. The process results in the formation of amorphous aluminum hydroxide that coats the fine cerium carbonate particles. After dehydration, drying, and calcination, cerium oxide doped with aluminum on the surface is obtained. Experimental results show that the intermediate ball-milling product is still predominantly hydrated cerium carbonate, and the formation of aluminum hydroxide inhibits the amorphization of cerium carbonate and its transformation into aluminum hydroxide. Under conditions where the aluminum doping does not exceed 10%, the calcined product retains a cubic fluorite structure.
All aluminum-doped cerium oxide powders exhibit a significantly enhanced polishing rate for ZF7 and K9 optical glasses compared to pure cerium oxide, proving that aluminum doping substantially improves the polishing performance of cerium oxide. The optimal aluminum doping level is 0.6%, with calcination carried out at 1000℃ for 2 hours, under which conditions the material removal rate is more than double that of pure cerium oxide.